Albemarle started producing alkali alkoxides in the early 1970s and has been continuously expanding the product line ever since.
They are broadly employed as bases in organic
synthesis of pharmaceuticals and fine chemicals. Typical reactions are
alkylation, arylations, solvolysis of esters and transesterifications.
Lithium Alkoxide
Albemarle offers lithium methoxide (powder and solution in methanol).
Magnesium Alkoxides
Albemarle offers magnesium
bis(2-ethylhexoxide), showing a high solubility in hydrocarbon solvents.
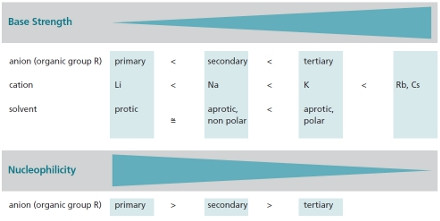
Properties
Reactivity
and selectivity can be varied by the organic group of the anion as well
as by the metal cation. Our products are available either as solids or
in solution, depending on the customer requirements.
Tailored reactivity and selectivity can be achieved
by varying either the “organic” group of the alkoxide anion or the metal
cation.
Influences on Base Strength and Nucleophilicity of Alkali Metal Alkoxides
Today organolithium amides are indispensable tools in modern, selective organic synthesis.
These bases are typically used for the formation of
enolates. Another important application is the ortho-selective
deprotonation of aromatic substrates.
Albemarle offers a great variety of different amides. These reagents include amides such as LiNH2, lithium diisopropylamide (LDA), and lithium hexamethyldisilazide (LHMDS, LHS).
Lithium Amides
Albemarle provides lithium amides starting with lithium amide (LiNH2) up to hindered amides such as lithium diisopropylamides (LDA), and lithium hexamethyldisilazide (LHMDS, LHS).
Organolithium amides are indispensable tools in
modern, selective organic synthesis. These bases are typically used for
the formation of enolates. Another important application is the
ortho-selective deprotonation of aromatic substrates.
Lithium
is the most reactive metal available. Albemarle provides this energy in
manageable forms such as organometallic reagents. These reagents can be
used to transform usually unreactive intermediates into highly reactive
starting materials for the synthesis of valuable substances in the
pharmaceutical and agrochemical industry, but also for the
polymerization of butadiene to yield synthetic rubber.
Our product range includes
- butyllithium (n-, sec-, iso-, tert-),
- hexyllithium
as well as other lithium organics like
- methyllithium,
- phenyllithium and
- lithium acetylide ethylene-diamine.
Butyllithium, Hexyllithium
The
most commonly used reagents are butyllithium and hexyllithium. They are
available on a large industrial scale. These reagents can be used as
strong bases in deprotonation reactions or for halogen/metal-exchange
reactions. The use of hexyllithium as a base avoids the emission of
butane.
Other Lithium Organics
Special
organolithium compounds like methyllithium, phenyllithium and lithium
acetylide-ethylene diamine are also available in multi-tons production
quantities. Phenyllithium can be used either as a base but also - like
the methyllithium and the lithium acetylide ethylene diamine complex -
for the introduction of the respective organic group.
- Methyllithium
- Phenyllithium
- tert-butyllithium
- Lithium acetylide
Experience and History
Albemarle is
one of the world's leading manufacturers of lithium-based compounds and
an innovative developer of metal-based fine chemicals for specialty
applications.
For many years now, organolithium compounds have
been straightforward reagents for organic syntheses. In the 1960s
butyllithium, as one of the most prominent products in this group,
started its industrial career as an initiator of the anionic
polymerization of alkenes in the synthetic rubber industry.
Some decades later, pharmaceutical companies
discovered the properties of butyllithium for their own syntheses, which
allows for these versatile reagents to be used in the metalation of
organic substrates. More and more companies are now equipped to use
butyllithium and other organolithium compounds, which increases their
technical capabilities of doing organic syntheses.
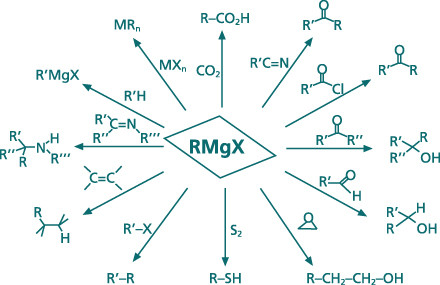
Grignard Compounds / Organomagnesium Compounds
An
expert for air-sensitive organometallic reagents, Albemarle offers a
fascinating range of organomagnesium compounds. Our existing product
portfolio covers a broad range of different alkylated- and arylated
magnesium compounds, which are useful tools for C-C bond-forming
reactions or deprotonation reactions. Organomagnesium compounds are
broadly employed in pharmaceutical, flavor and fragrances, polymer and
agrochemical applications.
Grignard Compounds / Organomagnesium Compounds
Dibutylmagnesium
Dibutylmagnesium
is used as a metalating agent for amines, alcohols, and carboxylic
acids as well as a reagent for the synthesis of Ziegler-Natta catalyst
systems and a modifier of anionic polymerization initiation.
While already offering a wide range of products,
Albemarle is also constantly developing new and innovative ones. Most
importantly, Albemarle is able to support the specific, unique needs of
each of its customers because of its extensive knowledge and experience
in the areas of organolithium and organomagnesium chemistry. This
experience includes both industrial and laboratory scale applications.
Grignards
Purchasing
ready-made Grignard solutions instead of producing them in-house helps
avoid the well-known potential hazard of the Grignard formation reaction
(accumulation, sudden start of reaction, high evolution of heat) and
the handling of occasionally highly toxic halogenated hydrocarbons (e.g. methyl bromide), additionally freeing up reactor
capacity for higher value products.
Our Experience - Your Advantage
We
are a specialty chemicals manufacturer and recognized expert in the
field of air-sensitive organometallic reagents for more than forty
years, started to produce and market Grignard compounds at about 1980.
Not only has it kept extending the product range
ever since; recently it introduced a new class of organomagnesium
compounds to perform halogen/metal exchange reactions, the
TurboGrignards.
TurboGrignards
Besides
C-C coupling reagents Albemarle offers a new class of organomagnesium
compounds for halogen-metal exchange reactions: the TurboGrignards. A
mixture of secondary alkyl-magnesium chloride with LiCl in THF combines
high reactivity with high functional group-tolerance in halogen-metal
exchange reactions.
Grignards in Green Solvents: 2-Methyltetrahydrofuran
Albemarle
offers various organomagnesium compounds in 2-MeTHF. These solutions
are typically higher concentrated compared to standard THF solutions and
are even more crystallization-stable (< -10 °C).
2-MTHF provides a lot of advantages in the customer process application.
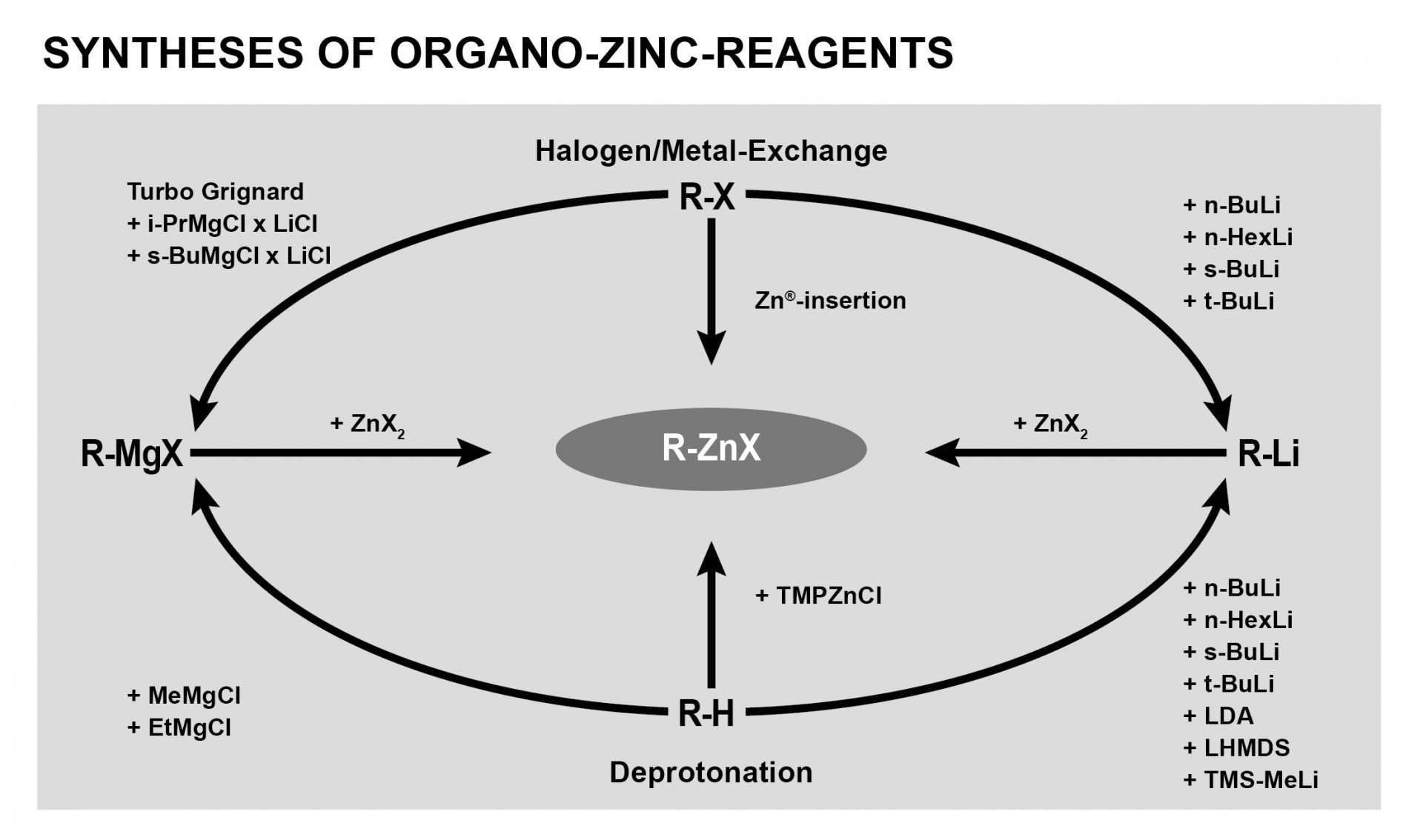
Toolbox for Organozinc Chemistry
The
very useful application of organozinc reagents in C-C cross-coupling
reactions was honored in 2010 by awarding the Nobel Prize to Ei-ichi
Negishi, Akira Suzuki, and Richard H. Heck. The demands of today’s API
and natural product synthesis require selective and reactive building
blocks. The production department in any pharmaceutical or chemical
company must also meet safety and environmental regulations as well as
operate in a profitable manner.
Organozinc reagents meet all of these requirements.
In contrast to lithium and magnesium organometallics, the corresponding
zinc reagents exhibit a high degree of chemo selectivity and are
tolerated by a large number of functional groups.
Even though organozinc reagents do not exhibit a
high reactivity towards most functional groups, in the presence of
transition-metal catalysts they easily undergo C-C coupling reactions
with an electrophile in a Negishi protocol.
The application spectrum of organozinc reagents is
comparable to that of boronic acid derivatives used in Suzuki coupling
protocols or organotin compounds in Stille coupling protocols.