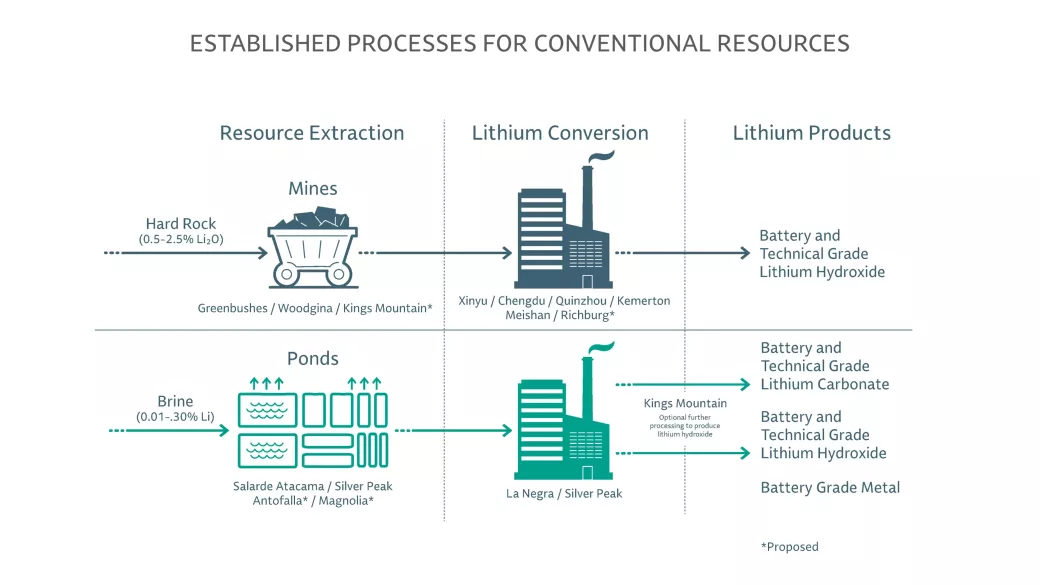
Whether brine or hard rock, production of the extracted lithium concentrates into technical or battery grades of lithium hydroxide or lithium carbonate is a critical part of the value chain for our customers. From chemical recovery of lithium brines to crushing, roasting and acid leaching of lithium ores in spodumene, Albemarle’s technical and chemical process know-how delivers consistent, high performing, low-cost lithium to power the world for generations to come.
Lithium Hydroxide
This fine crystalline powder is used as a precursor for the manufacturing of cathodes with mid- and high-nickel chemistries that drive higher energy density and performance. The preferred feedstock is hard rock spodumene; however, the salt can also be produced from the caustification reaction of lithium carbonate with calcium hydroxide.
Lithium Carbonate
The lithium salt of carbonic acid is a widely used precursor for compounds in the cathode and electrolyte of the battery. Typically produced from brine pools, lithium carbonate is predominately used in applications for a lithium phosphate (LFP) cathode but can also be used in mid-nickel chemistries or as an intermediary for the production of lithium hydroxide.
Spodumene Concentrate
A high lithium ore, containing approximately 6% lithium, is the main raw material used in the production of lithium salts (lithium hydroxide or lithium carbonate) from hard rock sources.